Abstract
Background: Hematological malignancies contribute a significant economic burden to health care systems. Therefore, to improve health care system planning, this Canadian study analyzed real-world data to estimate phase-specific costs and health care resource utilization for patients with hematological malignancies.
Methods: A cohort of adult patients diagnosed with hematological malignancies (diffuse large B-cell lymphoma [DLBCL], Hodgkin lymphoma [HL], follicular lymphoma [FL], chronic lymphocytic leukemia [CLL], multiple myeloma [MM], acute myeloid leukemia [AML], acute lymphocytic leukemia [ALL], and others) were identified between 2003-2014 from the Ontario Cancer Registry (OCR) and linked to treatment data from Cancer Care Ontario and other provincial administrative health care databases. Health system costs and resource utilization were determined for four defined phases of care in which index date was defined as the diagnosis date, including: pre-diagnosis (90 days prior to index date), initial treatment (index date to 6 months), follow-up (end of treatment phase to beginning of end-of-life or completion of cohort follow-up) and end-of-life (last 6 months of life for patients who died). Costs (Canadian dollars in 2014) and resource utilization were normalized to 30-days.
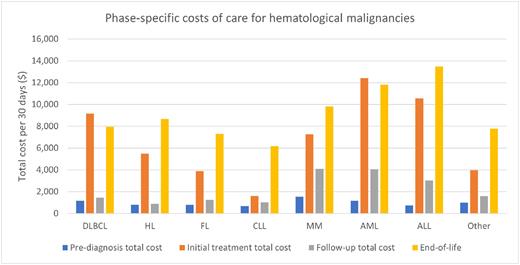
Results: There were 35,556 individuals (45% females and 55% males) diagnosed with hematological malignancies. The median age of diagnosis was 64 (IQR: 53-74) years. The median follow-up was 1,874 (IQR: 1,232-2,814) days. At follow-up completion, 26,092 (73.4%) individuals were alive. The median survival time among those who died was 1,005 (568-1,682) days. The phase-specific costs were highest overall in AML and ALL for the initial treatment and end-of-life phases; the costs were $1,169 (±3,230) and $733 (±1,787) for pre-diagnosis, $12,424 (±8,181) and $10,565 (±9,823) for initial treatment, $4,051 (±6,152) and $3,033 (±5,991) for follow-up, as well as $11,815 (±13,973) and $13,484 (±12,039) for end-of-life for AML and ALL, respectively. The phase-specific costs of care for DLBCL and HL were $1,175 (±2,267) and $813 (±1,541) in the pre-diagnosis phase, $9,166 (±5,581) and $5,474 (±3,485) in the initial treatment phase, $1,462 (±2,757) and $895 (±1,619) in the follow-up phase, and $7,966 (±7,104) and $8,679 (±9,087) in the end-of-life phase. For MM, the costs were $1,537 (±3,011) in pre-diagnosis, $7,254 (±6,870) in initial treatment, $4,100 (±4,217) in follow-up, and $9,820 (±8,294) in end-of-life. In comparison, the costs of care for FL and CLL were less in the initial treatment phase but remained elevated in the end-of-life phase; the costs were $775 (±1,798) and $674 (±1,902) in pre-diagnosis, $3,884 (±3,997) and $1,610 (±3,345) in initial treatment, $1,253 (±1,744) and $1,010 (±1,827) in follow-up, and $7,310 (±6,852) and $6,189 (±6,413) in end-of-life phases. Across the phases, the key cost driver was inpatient care. In particular, the increased costs due to inpatient care for the initial treatment and end-of-life phases, respectively, were: $3,864 (±5,013) and $5,045 (±5,908) for DLBCL; $2,384 (±3,712) and $6,441 (±8,129) for HL; $2,131 (±3,502) and $5,146 (±5,612) for FL; $2,435 (±3,860) and $4,498 (±5,582) for CLL; $3,798 (±4,847) and $5,026 (±6,788) for MM; $9,330 (±6,985) and $7,941 (±9,063) for AML; and $8,133 (±8,178) and $10,144 (±10,780) for ALL. The second largest contributor to the cost of care, particularly in the initial treatment phase, was the cost of cancer treatment medication; $3,115 (±1,236) for DLBLC, $1,132 (±766) for HL, $2,851 (±1,755) for FL, $1,069 (±1,549) for CLL, $1,602 (±1,877) for MM, $843 (±1,429) for AML, and $494 (±756) for ALL. Of note, the cost of cancer treatment medication remains elevated for MM across the phases after diagnosis; $1,602 (±1,877) in initial treatment, $1,622 (±2,160) in follow-up, and $2,078 (±2,697) in end-of-life.
Conclusions: In general, the mean total costs were highest in the initial treatment and end-of-life phases, and lowest in the pre-diagnosis and follow-up phases of care for all hematological malignancies, following a U-shaped pattern. Inpatient care was the cost driver across all phases. These findings can help allocate appropriate resources throughout the different phases of cancer care for hematological malignancies, with particular focus on improving access to palliative-care at end-of-life.
Disclosures
Singh:Sanofi: Other: Employment by An Immediate Family Member; Advanced Accelerator Applications/Novartis: Honoraria; IPSEN: Honoraria; Novartis: Other: Research funding for institution.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal